How it Works
Who does KerloSense Help?
KerloSense serves sponsors, sites, and site management organizations (SMOs) by offering an all-in-one solution for global site identification, study start-up, and collaboration.
Sponsors gain direct access to qualified sites, sites find new study opportunities without any fees, and SMOs can efficiently manage their network, making it easier than ever to advance clinical research.

Negotiate your own Budget
Negotiate directly with sponsors, empowering you to secure fair compensation and prioritize resources effectively

Track Study Progress
Manage completed visits and procedures seamlessly, integrating them directly into the invoicing system to ensure accurate and efficient billing

Secure Communication
Allowing verified sponsors and sites to collaborate efficiently and confidently in a protected environment
Site Identification
Site Search makes it easier for sponsors and CROs to connect with the right clinical research sites.
Through our centralized portal, you can filter a global network of sites and PIs based on criteria like therapeutic focus, study phase, geographic location, and past trial experience. Once you’ve identified potential matches, you can send direct invitations to sites that meet your study’s requirements — no middle-men involved.
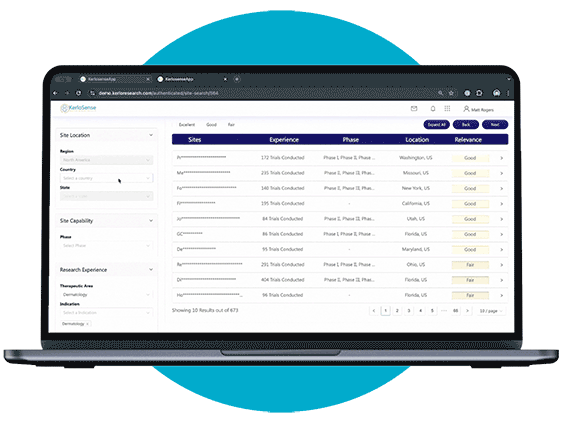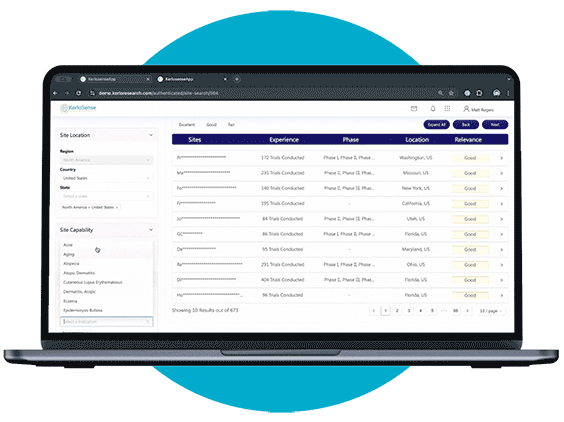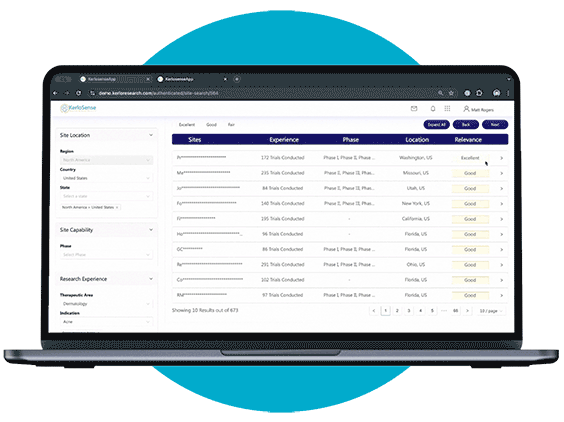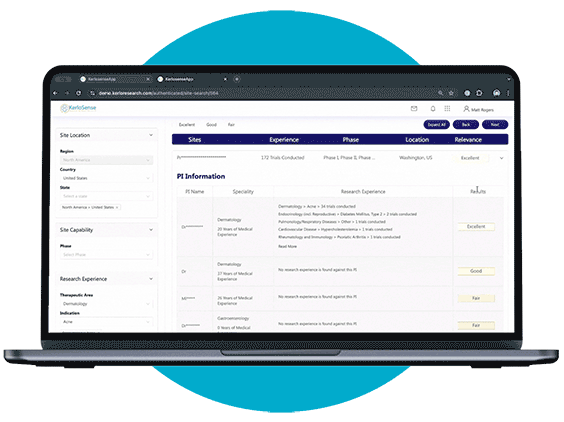
What sets KerloSense apart is its transparency. You’ll see when sites engage, track interest, view PI profiles and capabilities — allowing you to make informed decisions throughout the site selection process.
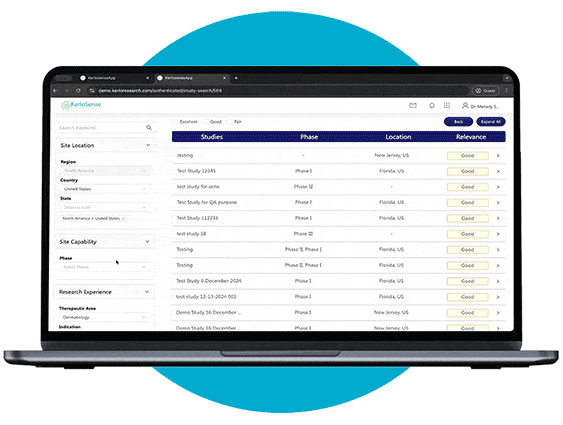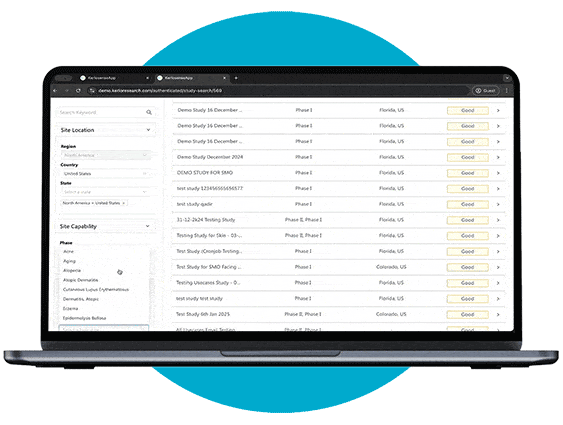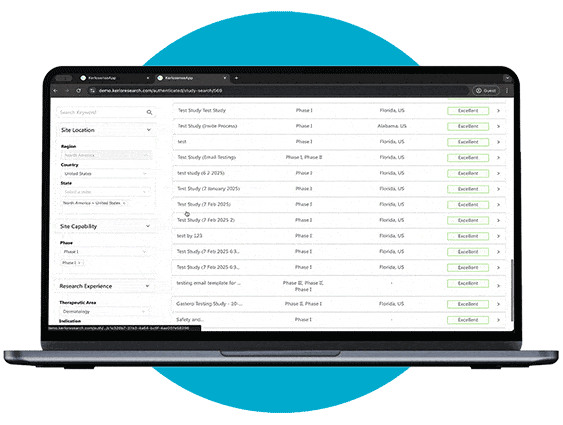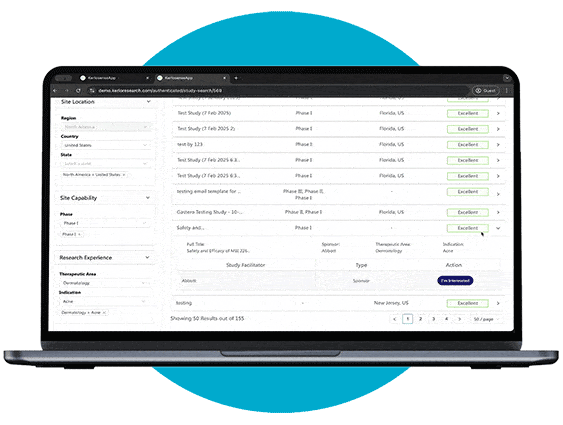
Study Identification
Through our portal, sites can browse available studies and filter opportunities based on their capabilities, interests, and experience.
When a study aligns with their expertise, they can express interest directly to the study owner, sharing their Principal Investigator’s (PI) information. The portal also allows users to track responses and see when their PI’s details have been viewed, creating a streamlined and transparent process for securing study opportunities.
Why Choose Us?

Seamless clinical trial recruitment and participant engagement.
Decentralized trials with virtual trials, telemedicine, and eConsent.

Compliant with ICH guidelines, FDA guidelines, and GCP standards.
For Sponsors & Researchers
Our Platform Supports:
01
Clinical trial management and design optimization.
02
Data-driven clinical trial monitoring and quality assurance.
03
Advanced analytics powered by AI and real-world evidence insights.

For Participants
Take part in transformative clinical studies with assurance of safety and transparency.
User-friendly informed consent processes.
Participation in innovative and ethical clinical research studies.

Seamless Site Management Awaits!
Seamless site management starts here! Connect with the right studies and streamline operations effortlessly.
